MOLECULAR DIAGNOSTICS
Molecular diagnostics is based on analyses of biological markers in the genome and proteome by applying molecular biology to medical testing and diagnostics and thus offers the prospect of personalized medicine such as infectious disease, oncology, pharmacogenomics, etc. for the patient’s thoughtfulness. Obviously molecular tests are sustained in clinical chemistry in which medical tests on bodily fluids are central, especially blood and urine.
The generation of viable biological markers microarrays is, though, challenging. For instance, current antibody-based optical microarrays are commonly based on sandwich assays in which antigen binding to the immobilized antibody is detected through the use of a secondary, labeled, antibody. Though sensitive, this approach is laborious and requires a specifically-labeled secondary antibody for every antigen of interest. Labeling protocols are potentially perturbative, time-consuming, and can lead to high background signals. Label-free detection assays, such as those based on plasmon resonance or QCM, offer, typically, nanomolar detection limits but require the use of sophisticated and expensive pieces of equipment and are not readily applied in any high throughput or practical personalized setting.
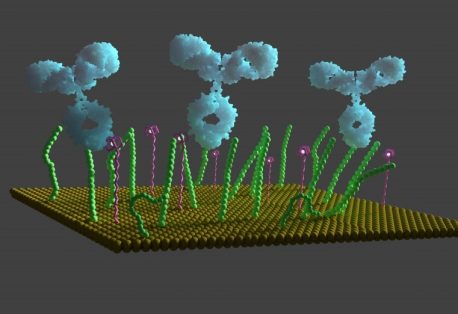
Within this body of knowledge the understanding and manipulating chemistry surface recognition, ubiquitous in nature, enables man to modulate the function and optimize recognition with potentially powerful applications in new methods of therapeutics and diagnostics. Nonetheless, in order to reach higher levels of sensitivity and to move to a detection format which is both potentially cheap, portable, and multiplexable, label-free electrical detection methods, those based on time-dependent approaches such as capacitance and impedance spectroscopic (faradaic and non-faradaic) are exceedingly powerful and sensitive. Assays are generated by controllably immobilizing receptive biomolecules (typically antibodies, nucleic acids, or peptides) on electrodes and converting the target protein binding event into a measurable electrical signal. One of the most sensitive and powerful means of doing this is by the use of time-dependent electroanalytical techniques such as capacitance and/or impedance spectroscopies optimized in frequency.
Impedance derived electroanalytical assays are inherently spectroscopic (frequency-resolved) and potentially exceedingly sensitive indicators of interfacial change (such as target binding with an appropriate receptor). In general, the impedance and capacitance complex functions are just one form of a more general terminology known as immittance (complex functions). Accordingly, all of these functions are associated with particular classes of immittance spectroscopies (impedance, capacitance, modulus, etc.). Historically, because the complex impedance function is the most used, all other immittance spectroscopies are said to be impedance-based methods. Therefore, we introduce the use of a portfolio of mathematically derived immittance functions and related components capable, from the same raw data sets, of enabling increased assay sensitivity and markedly shorter assay times in comparison to traditional impedance analyses. The methodology, applied to faradaic (redox probe amplified) and non-faradaic assays, requires no equivalent circuit analysis or previous assumption based on responsiveness. Its focus is to optimize analytical potency and to enable the user to select and apply the most frequency-optimized reporter of interfacial change and to, thereafter, run rapid (optimized) analyses at single frequencies. This approach, then, is not only capable of sampling many more interfacial changes that would necessarily be probed by a standard impedance or capacitance assay but also does so with no prior assumptions about what function (interfacial resistance, complex dielectric, complex capacitance, phase, etc.) is optimal. It also frees multiplexed analyses from the confines of having to use the same sampling or fitting procedure for each electrode regardless of the receptor, target, or chemistry present. Furthermore, if specific receptor-target interactions are found to be associated with a calibratable change in only specific functions, it may be possible to achieve multianalyte detection at single electrodes (i.e. without the need for an array).
